EU backs AstraZeneca vaccine as blood clot reviews continue
Europe's drug regulator reiterated today that the benefits of AstraZeneca's COVID-19 vaccine outweigh any risks, as part of a detailed guidance from ongoing reviews into rare blood clots to help individual nations determine the shot's use.
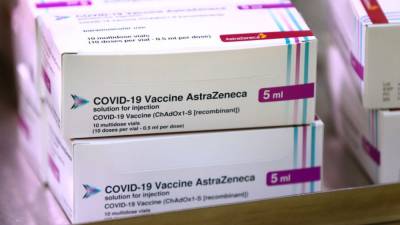
The renewed backing comes after several countries in the European Union and worldwide have limited the use of the vaccine, known as Vaxzevria, while Denmark has stopped its use altogether, after possible links to clotting issues were confirmed.
The interim analysis by a committee of the European Medicines Agency (EMA) determined that serious side effects of rare blood clots are likely to occur in 1 out of 100,000 vaccinated people, the regulator said in a statement.