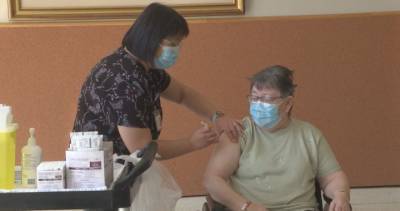
US expert panel recommends authorising Johnson & Johnson vaccine
A US panel of independent experts has voted unanimously in favour of recommending Johnson & Johnson's one-dose Covid-19 shot for emergency approval.
The decision clears the way for a third vaccine to soon begin being distributed in the country hardest hit by the global pandemic.
The committee's 22 members were convened by the Food and Drug Administration and included leading scientists as well as consumer and industry representatives.
Although their recommendations are not binding, they are usually followed. An emergency use authorisation (EUA) will now likely follow, probably in the coming days, making the J&J vaccine the third to be licensed in the United States after the Pfizer-BioNTech and Moderna inoculations were provisionally