Pfizer requests U.S. emergency use for its COVID-19 vaccine in kids aged 12-15
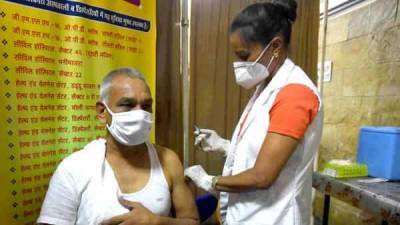
Pfizer and its German partner BioNTech said on Friday they have requested the U.S. Food and Drug Administration to expand the emergency use of their COVID-19 vaccine in adolescents aged 12 to 15.In March, the drugmakers said the vaccine was found to be safe, effective and produced robust antibody responses in 12- to 15-year olds in a clinical trial.
Pfizer says COVID-19 vaccine is 100% effective in protecting kids aged 12-15 in trial Whether COVID-19 vaccines work and are safe to use on children is one of the big questions drugmakers are trying to answer.
Inoculating children and young people is considered a critical step toward reaching “herd immunity” and taming the pandemic, according to experts.The companies plan to request similar.