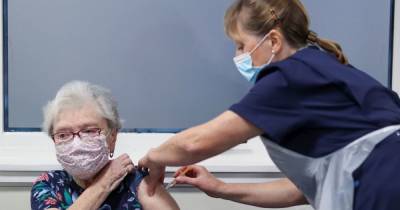
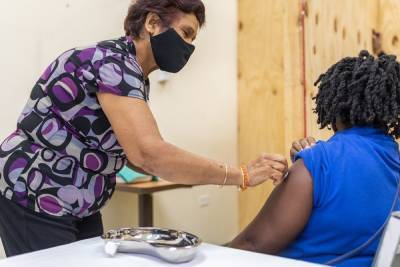
Pfizer, BioNTech seek COVID vaccine authorization for kids
LONDON – Pfizer Inc. and BioNTech have submitted a request to the European drug regulator for the approval of their coronavirus vaccine to be extended to include children 12 to 15 years old, in a move that could offer younger and less at-risk populations in Europe access to the shot for the first time.
In a statement Friday, the two pharmaceuticals said their submission to the European Medicines Agency is based on an advanced study in more than 2,000 adolescents that showed their vaccine to be safe and effective.
The children will continue to be monitored for longer-term protection and safety for another two years. BioNTech and Pfizer have previously requested their emergency use authorization with the U.S.