fox29.com
81%
560
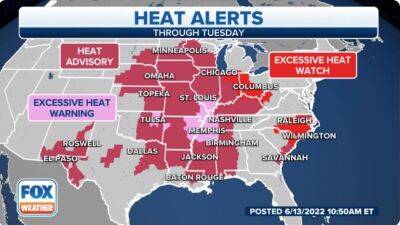
106 million Americans in 24 states at risk as dangerous heat wave expands
More than 106 million Americans in 24 states are under a heat alert.(FOX Weather) The National Weather Service says heat is expected to persist for the next few days, with well-above-average to record temperatures expected from the central and southern Rockies across the Plains and into the lower and mid-Mississippi, Tennessee and lower Ohio valleys.And as the heat index is expected to easily reach above 100 degrees in many parts of the country, Heat Advisories, Excessive Heat Warnings and Excessive Heat Watches have been posted across most of the eastern Plains states, the lower and mid-Mississippi Valley, the Ohio Valley the lower Tennessee Valley and the central Gulf Coast.The NWS says a strong area of high pressure will begin to build over the Tennessee Valley, which will also allow the heat to begin to build up in the Carolinas.WHAT IS THE 'FEELS LIKE' TEMPERATURE?The forecast high temperatures on Monday, June 13.(FOX Weather) Check out these high temperatures in the South on Monday.It will get into the mid- to upper 90s across the region from Dallas and Houston and along the Gulf Coast into the Mississippi and Tennessee valleys, as well as the Southeast.Nashville, Tennessee, will see a high temperature of about 100 degrees, but it will feel even hotter when you factor in the heat index.It will feel like 110 degrees or higher across the region on Monday.(FOX Weather) Even though the temperatures across the region will be at or near 100 degrees, it will feel even hotter.Dallas and Houston will feel like it's 103 degrees, and Little Rock, Arkansas, and Alexandria, Louisiana, will feel like 105 degrees.Nashville and Tuscaloosa, Alabama, will feel like it's about 100 degrees.HOW THE WEATHER YOU'RE ACCUSTOMED TO AFFECTS