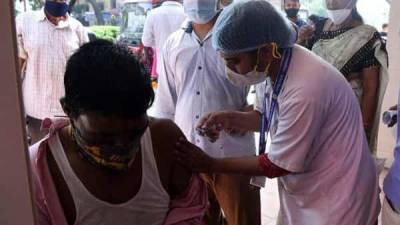
J&J COVID-19 booster shot needs more evidence, FDA analysis suggests
WASHINGTON - The Food and Drug Administration said Wednesday it is wrestling with whether and when recipients of the single-shot Johnson & Johnson COVID-19 vaccine need another dose — at six months or as early as two months.In an online review, FDA scientists didn’t reach a firm conclusion, citing shortcomings with J&J’s data, including little information on protection against the extra-contagious delta variant of the coronavirus.The review comes ahead of meetings Thursday and Friday when an FDA advisory panel will recommend whether to back booster doses of both the J&J and Moderna vaccines.
That’s one step in the government’s vaccine review process: Next week, the FDA will make a final decision on authorizing those boosters and then the.