FDA signals apprehension about COVID-19 vaccine booster shots
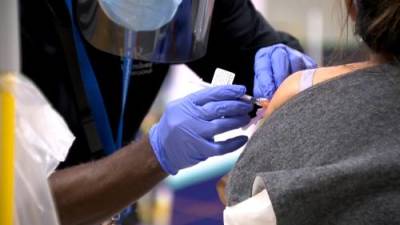
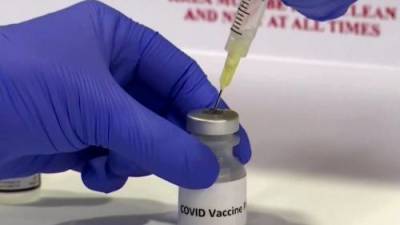
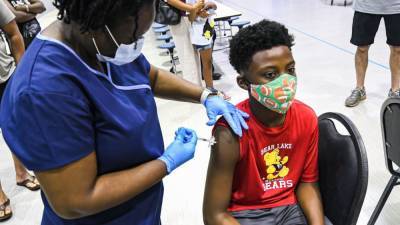
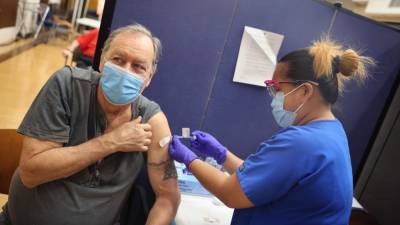
Tomorrow's VRBPAC (Vaccines and Related Biological Products Advisory Committee) meeting will consider Pfizer/BioNTech's application for a COVID-19 vaccine booster dose, but the Food and Drug Administration (FDA) has signaled in a new report today that booster doses, though effective, are not indicated at this time for most Americans.The FDA said while some observational studies do show Pfizer's vaccine has waning protection against the Delta (B1617.2) variant, other studies do not.Pfizer's mRNA-based vaccine is the only FDA-approved COVID-19 vaccine for use in the United States, and the company's application is for a third booster dose to be given 6 months after the initial two-dose series.