FDA: Novavax COVID vaccine effective, but cardiac questions remain
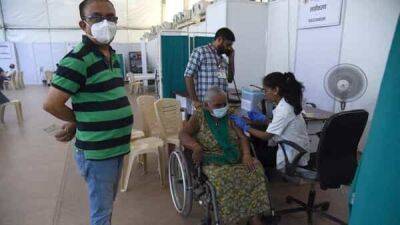
In a detailed review ahead of next week's advisory committee meeting, Food and Drug Administration (FDA) staff today said the Novavax COVID-19 vaccine will likely provide meaningful protection against the Omicron variant, but they raised concerns about the potential for rare cardiac conditions after vaccination.Cardiac issues raised with other vaccinesThe FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) is slated to consider emergency use authorization (EUA) for the Novavax vaccine on Jun 7.The vaccine is a recombinant nanoparticle protein-based product that contains an adjuvant and is given in two doses 3 weeks apart.
The vaccine is made on a more traditional production platform, raising hopes of increased vaccine uptake among Americans who have shied away from newer mRNA vaccines.
The vaccine is already used in other parts of the world.In an 80-page review of the vaccine's efficacy and safety, the FDA combed through trials that included 30,000 patients and were conducted before the Delta and Omicron surges.
Last June, a phase 3 trial found that the vaccine had 90% overall efficacy and was well tolerated, with few serious and adverse events.