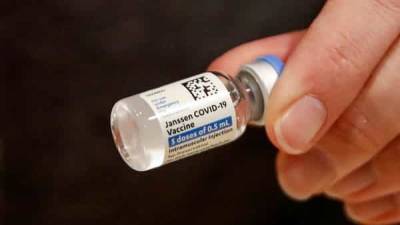
EU drug regulator prepares to issue advice on J&J COVID shot
LONDON – Experts at the European Medicines Agency are preparing to present the conclusions of their investigation later on Tuesday into possible links between the Johnson & Johnson coronavirus vaccine and very rare cases of unusual clotting disorders detected in the U.S.
Last week, J&J halted its European rollout of its one-dose vaccine after the U.S. Food and Drug Administration recommended officials pause its use while the rare blood clot cases are examined.
Officials identified six cases of the highly unusual blood clots among nearly 7 million people who were immunized with the shot in the U.S.
J&J advised European governments to store their doses until the EU drug regulator issued guidance on their use; widespread use of the shot in