Canada says COVID-19 boosters may be needed but no approval request from Pfizer yet
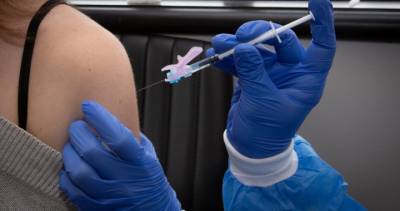
COVID-19 vaccine, and it is currently studying the vaccine’s duration of protection, the Health Ministry said in a statement on Friday.“Health Canada has not received a submission from Pfizer for the approval of a COVID-19 booster shot.
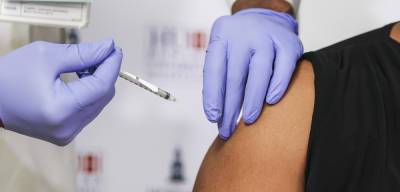
Should a submission be received, Health Canada will evaluate the data provided,” the statement said. Pfizer developing booster shot to combat COVID-19 Delta variant “The duration of protection from one or two doses of COVID-19 vaccines is currently being studied.”Pfizer and BioNTech plan to ask U.S.
and European regulators within weeks to authorize a booster dose of its COVID-19 vaccine, based on evidence of greater risk of infection six months after inoculation and the spread of the highly contagious.